 |
| |
|
|
|
|
| 引言 |
 友善列印 友善列印 |
感謝國內十餘位教授於繁忙的公務外熱心提供資料,並感謝中研院化學所提供製作網頁人力。
二十一世紀之良性社會發展倚靠公民參與精神之提昇。
目前資源共享網雛型已成,歡迎大家提供資料與改進方向,讓資源共享的精神促進科研之推廣與發展。 |
| 2006. 7 |
|
綠色化學的核心內容之一是採用“原子經濟 (atom economy)”的觀念,此概念最早是由美國Stanford大學的Barry M.Trost教授提出的,相對於傳統的化學反應的產率觀念只計算單一反應物轉化到所要產物的比率,它考慮的是在化學反應中全部原料的原子進入到所要產物之中的比率。這種標準即是要求盡可能地減少廢物產生,也節約不可再生的原料資源。“原子經濟”的重要性目前己被普遍承認,Trost教授因此獲得了1998年美國“總統化學挑戰獎”的學術獎章。
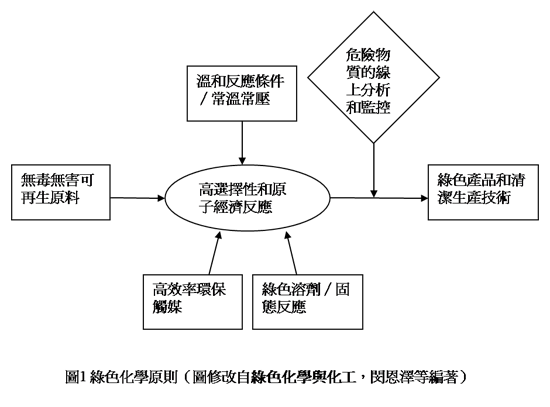
要實現化學反應的高原子經濟性,就需要開發新的反應途徑。根據P. T. Anastas所提出的綠色化學12條指導原則,其研究重點是朝採用無毒無害的原料、綠色化學反應途徑、高效率環保觸媒、綠色溶劑和產品等方向前進,如圖1所示。其中使用催化反應代替化學計量反應是一種很好的途徑,因為催化劑可以加速反應速率,又不在反應中消耗,可以重複使用,百分之六十的化學製品在生產過程中都必需使用到觸媒。異相催化(heterogeneous catalysis)是指催化劑與反應物在不同相(固相、液相、氣相等)下所進行的催化反應,由於反應後產物與催化劑可以很容易的經由沖提或過濾分離,所以使用異相觸媒當然是執行綠色化學的重要手段之一,也是工業界最歡迎的製程。
我們生活中最常見的異相催化反應的例子是汽車廢氣排放前經過的「觸媒轉化器」,可以把有毒的CO, 碳氫化合物, NO, NO2等經負載在陶瓷材料上的貴金屬催化劑轉化成無毒的H2O, CO2與N2,再排放到空氣中。另外,二氧化鈦光觸媒可以經由照光產生電子/電洞對,進而分解吸附在其表面的污染物。這些都是異相觸媒材料被應用到毒性物質與污染物的去除的例子。
在綠色化學領域,開發新觸媒材料和新的催化製程以取代傳統生產反應技術一直是科學界重要的研究方向,比如以固體酸觸媒取代化工製程常用的高腐蝕性硫酸、氫氟酸,以減少或避免廢液的產生。在新能源的開發方面,開發異相觸媒材料在再生能源的製備是最近幾年極為重要的課題。
為維持社會永續發展,綠色催化技術有必要積極發展下列領域:
- 發展有效利用太陽光能的催化材料和技術。
- 發展利用可再生原料為反應物的合成技術。
- 發展清潔和高效率的催化技術來取代現有高汙染的製程。
|
| |
|
| 書籍 |
 |
Principles and Practice of Heterogeneous Catalysis by John Meurig Thomas, W. John Thomas —
當代異相催化的理論、技術及應用。 |
 |
Catalytic Science Series - Vol. 1 Envitronmental Catalysis — Edited by F J J G Janssen & R A van Santen (EindhovenUniversity of Technology, The Netherlands) Apr 1999
This book brings together highlights of a theme which is growing in interest: the creation of a sustainable society using catalysis as the main tool. Catalysts play key roles in the production of clean fuels, the conversion of waste and green raw materials into energy, clean combustion engines including control of NOx and soot production and reduction of greenhouse gases, production of clean water and of polymers, as well as reduction from polymers to monomers. Catalysts are also of prime importance in the developing H2 and syngas production technology, aimed at producing clean fuels for the coming decades. And catalysts can be recycled.
本書探討如何利用異相觸媒來創造一個永續社會,探討的觸媒應用包括:生產乾淨能源、再生能源、廢氣及溫室氣體的處理等。 |
 |
Catalytic Control of Air Pollution: Mobile and Stationary Sources — Editors: Ronald G. Silver, John E. Sawyer, Jerry C. Summers Washington : American Chemical Society, 1992
Developed from a symposium sponsored by the Division of Colloid and Surface Chemistry of the American Chemical Society at the Fourth Chemical Congress of North America (202nd National Meeting of the American Chemical Society), New York, New York, August 25-30, 1991 |
| |
|
|
| A Few Research Directions in Green Heterogeneous Catalysis |
| Solar Energy Utilization |
 |
Photocatalysis: A Promising Route for 21st Century Organic Chemistry ─ Chem. Commun., 2007, 3425. |
 |
Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications ─ Chem. Rev., 2007, 107, 2891. |
 |
A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications ─ Solar Energy Materials & Solar Cells, 2006, 90, 2011. |
 |
Photocatalysis with Organized Systems for the Oxofunctionalization of Hydrocarbons by O2 ─ Chem. Rev., 2002, 102, 3811. |
 |
A Review and Recent Developments in Photocatalytic Water----Splitting Using TiO2 for Hydrogen Production ─ Renewable and Sustainable Energy Reviews, 2007, 11, 401. |
 |
Photocatalytic Conversion of Methane ─ Chem. Soc. Rev., 2008, 37, 1592. |
 |
An Efficient Bicompoment TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with A Side-by-side Dual Spinneret Method ─ Nano Letters, 2007, 7, 1081. |
 |
Effect of Calcination Temperature on the Structure of a Pt/TiO2 (B) Nanofiber and Its Photocatalytic Activity in Generating H2 ─ Langmuir, 2008, 24, 9907. |
 |
Large-scale Photochemical Reactions of Nanocrystalline Suspensions: A Promising Green Chemistry Method ─ Organic Letters, 2006, 8, 2615. |
| |
|
|
| CO2 as the Raw Material and the Reaction Medium |
 |
Utilisation of CO2 as a Chemical Feedstock: Opportunities and Challenges ─ Dalton Trans., 2007, 2975. |
 |
CO2 Reforming of CH4 ─ Catalysis Reviews, 1999, 41, 1. |
 |
Highly Efficient Supramolecular Photocatalysts for CO2 Reduction Using Visible Light ─ Photochem. Photobiol. Sci., 2007, 6, 454. |
 |
Metal-coated Nanoscale TiO2 Catalysts for Enhanced CO2 Photoreduction ─ Green Chem., 2005, 7, 667. |
 |
Oxidation Reactions in CO2: Academic Exercise or Future Green Processes? ─ Environ. Sci. Technol., 2003, 37, 5289. |
 |
Supercritical Carbon Dioxide as a Green Reaction Medium for Catalysis ─ Acc. Chem. Res., 2002, 35, 746. |
 |
Organic Synthesis Using Enzymes in Supercritical Carbon Dioxide ─ Green Chem., 2004, 6, 440. |
 |
Friedel-Crafts Alkylation of Anisole in Supercritical Carbon Dioxide: A Comparative Study of Catalysts ─ Org. Process Res. Dev., 2005, 9, 451. |
 |
Synthesizing Silver Halide Nanoparticles in Supercritical Carbon Dioxide Utilizing A Water-in-CO2 Microemulsion ─ Chem. Commun., 2000, 2353. |
| |
|
|
| Solid Acid Catalysis |
 |
Solid Acids for Green Chemistry ─ Acc. Chem. Res., 2002, 35, 791. |
 |
Acid Catalysts in Industrial Hydrocarbon Chemistry ─ Chem. Rev., 2007, 107, 5366. |
 |
Water-tolerant Solid Acid Catalysts ─ Chem. Rev., 2002, 102, 3641. |
 |
Efficient Utilization of Nanospace of Layered Transition Metal Oxide HNbMoO6 As A Strong Water-tolerant Solid Acid Catalyst ─ J. Am. Chem. Soc., 2008, 130, 7230. |
 |
Methane Carbonylation with CO on Sulfated Zirconia: Evidence from Solid-state NMR for the Selective Formation of Acetic Acid ─ J. Phys. Chem. C., 2007, 111, 10624. |
 |
The Effect of Supercritical Fluids on Solid Acid Catalyst Alkylation ─ Ind. Eng. Chem. Res., 2002, 41, 2864. |
 |
Continuous iC4/C4= alkylation Under iC4 Supercritical Conditions Over K2.5H0.5PW12O40 and H-Beta Solid Acids ─ Ind. Eng. Chem. Res., 2004, 43, 6355. |
 |
Ionic Liquid and Solid HF Equivalent Amine-poly(Hydrogen Fluoride) Complexes Effecting Efficient Environmentally Friendly Isobutane-isobutylene Alkylation ─ J. Am. Chem. Soc., 2005, 127, 5964. |
 |
Heterogeneous Catalysis Using A Nanostructured Solid Acid Resin Based on Lyotropic Liquid Crystals ─ J. Am. Chem. Soc., 2004, 126, 1616. |
| |
|
|
| Greener Reaction Paths |
 |
“Designing Catalysts for Clean Technology, Green Chemistry and Sustainable Development” by John M. Thomas and Robert Raja — Annual Review of Materials Research, 315, 35, 2005 |
 |
Origins, Current Status, and Future Challenges of Green Chemistry ─ Acc. Chem. Res., 2002, 35, 686. |
 |
E Factors, Green Chemistry and Catalysis: An Odyssey ─ Chem. Commun., 2008, 3352 |
 |
Atom Efficiency and Catalysis in Organic Synthesis ─ Pure ppl. Chem., 2000, 72, 1233. |
 |
Recent Progress in Synthesis of Fine and Specialty Chemicals from Wood and Other Biomass by Heterogeneous Catalytic Processes ─ Catalysis Reviews, 2007, 49, 197. |
 |
Heterogeneous Catalytic Synthesis of Ethanol From Biomass-derived Syngas ─ Chem. Soc. Rev., 2007, 36, 1514. |
 |
Synthesis of Biodiesel Via Acid Catalysis ─ Ind. Eng. Chem. Res., 2005, 44, 5353. |
 |
ε-Caprolactam: New by-Product Free Synthesis Routes ─ Catalysis Reviews, 2001, 43, 381. |
 |
Fuels and Energy for the Future: The Role of Catalysis ─ Catalysis Reviews, 2004, 46, 247. |
| |
|
|
| Recent Developments in Catalyst Technologies Worthy of Noticing |
 |
Application of Combinatorial Technologies for Catalyst Design and Development ─ Catalysis Reviews, 2000, 42, 385. |
 |
Computational Modeling of Catalytic Reactivity ─ Molecular Simulation, 2007, 33, 327. |
 |
Catalysis in Ionic Liquids ─ Chem. Rev., 2007, 107, 2615. |
 |
Metal-organic Frameworks ─ Chem. Soc. Rev., 2003, 32, 276. |
 |
Alkylation, Hydrogenation and Oxidation Catalyzed by Mesoporous Materials ─ Catalysis Reviews, 2005, 47, 1. |
 |
Recent Advances and Future Aspects in the Low-temperature Conversion of Saturated Hydrocarbons ─ Catalysis Reviews, 2002, 44, 455. |
 |
Photochemistry on Metal Nanoparticles ─ Chem. Rev., 2006, 106, 4301. |
 |
Ordered Macroporous Bimetallic Nanostructures: Design, Characterization, and Applications ─ Acc. Chem. Res., 2008, 41, 244. |
 |
Nanoqueous Sol-gel Routes to Metal Oxide Nanoparticles ─ Acc. Chem. Res., 2007, 40, 793. |
 |
Carbon Nanofiber Supported Palladium Catalyst for Liquid-phase Reactions. An Active and Selective Catalyst for Hydrogenation of C=C Bonds ─ Chem. Commun., 2000, 1871. |
 |
TiO2 Nanotube-supported Ruthenium(III) Hydrated Oxide: A Highly Active Catalyst for Selective Oxidation of Alcohols by Oxygen ─ J. Catal., 2005, 235, 10. |
| |
|
|
|
| 重要網站 |
|
| 入門文章 |
|
| Conferences |
|
| Journal
|
|
| Special Issues
|
 |
“Catalytic Conversion of Renewables” Topics in Catalysis, Vol. 27 (1-4) 2004 — Guest editors: Herman van Bekkum, Pierre gallezot
This special issue of Topics in Catalysis was mainly aimed at publishing state-of-the-art research on the catalytic conversion of bio-based resources to high value-added chemicals and products. Our experience is that the mainstream of the catalysis community is not aware of recent progress made in this area. Indeed, besides well-known processes such as the catalytic hydrogenation of carbohydrates and vegetable oils, a very diversified chemistry is under development, often combining heterogeneous, homogeneous, and enzymatic catalysis to attain high yields of products via environmentally benign processes. We have chosen to focus on catalytic transformations of carbohydrates, fatty compounds derived from vegetable oils, and terpenes. |
| |
|
|
| |
| |
|
| |
|
聯絡我們|國科會化學中心 |
| 本站適用瀏覽器為IE 6.0以上;最佳瀏覽解析度為1024*768 Update:
2020-09-08
|
| Copyright©2006 國科會化學中心. All Rights Reserved |
|
|
 |



