 |
| |
|
|
|
|
| 引言 |
 友善列印 友善列印 |
感謝國內十餘位教授於繁忙的公務外熱心提供資料,並感謝中研院化學所提供製作網頁人力。
二十一世紀之良性社會發展倚靠公民參與精神之提昇。
目前資源共享網雛型已成,歡迎大家提供資料與改進方向,讓資源共享的精神促進科研之推廣與發展。 |
| 2006. 7 |
|
離子液體當作溶劑的應用
近年來,離子液體在化學上的應用非常廣泛。由於離子液體具有極低蒸氣壓、低熔點、高極性、不可燃性、耐強酸、高熱穩定性、高導電度、電化學性佳及較廣的液體溫度範圍( -96 ~ 400 ℃)等特殊性質,可替代一般所用之揮發性有機溶劑(volatile organic compounds VOCs),應用在化學合成,而且離子液體可在常壓下操作,不但可降低操作成本,且可消除VOCs對環境的污染,並可避免操作人員暴露於VOCs的風險,可再回收使用,所以離子液體有時被認為是一種新的綠色溶劑“green solvent”[1]。
離子液體是由陰離子及陽離子所組成的有機熔鹽,依不同組合方式,可超過一兆種。鹽類的熔點可高達 801 ℃或低到–96 ℃,所以為方便與高溫熔鹽做區分,通常把熔點低於 100 ℃的熔鹽稱為室溫離子液體(room temperature ion liquids-RTILs),簡稱為離子液體(IL),目前所發現的離子液體已超過 200 多種,常用的離子液體結構如圖一[2]。離子液體的陽離子包含有1-alkyl-3-methylimidazolium ([CnMIM]+, n為線性烷基碳的數目)、N-alkylpyridinium([CnPY]+)、tetraalkylammonium及tetraalkylphosphonium等陽離子,這些陽離子可結合不同的有機或無機的陰離子形成數目龐大的離子液體[3],常見的陰離子有hexafluorophosphate(PF6-)、tetrafluoroborate(BF4-)、trifluoromethylsulfonate(CF3SO3-)、bis[(trifloromethyl)sulfonyl]amide [(CF3SO2)2N]-、trifluoroethanoate(CF3CO2-)、ethanoate(CH3CO2-)及halide(Br-,Cl-,I-)。
雖然早在 1914 年Walden即首先報導低溫的離子液體ethylammonium nitrate[4],接下來於 1951 年Hurley首先合成室溫離子液體N-ethylpyridinium bromide- aluminium chloride[5],但一直到 1970 年代,Osteryong和Willkers成功製備出chloroaluminate melts[6],從此離子液體被大量應用於電化學、反應介質及催化劑。 1992 年,Wilkes等人發展出一係列咪唑(imidazolium)陽離子及BF4-、PF6-等陰離子組成的離子液體[7],此類離子液體在空氣及水中相當穩定,使得這些類離子液體的應用引起廣大重視[8],之後離子液體的發展大多以咪唑鹽類為主,進而發展出含DNA離子液體[9]、適合電化學的兩性離子液體[10]、磁性離子液體[11]及以胺基酸作為陰離子的離子液體等特殊功能的離子液體[12],近年來離子液體的研究趨勢往功能性上發展。
離子液體的物理性質:
- 親水性:離子液體的親水性主要是取決於陰離子的結構,對水溶解度趨勢如圖一[3],另外陽離子碳鏈愈長親水性愈差。
- 酸鹼性:一般離子液體可由陰離子部分判斷其酸鹼性,如表一[13],因此可藉由陰離子的部分來調控溶劑的酸鹼度,而不必再加入額外的酸或鹼。
- 熔點:陽離子的對稱性愈低,會影響晶體的堆疊性,使熔點降低,而分子間的氫鍵會使熔點提高,常用雙烷基咪唑鹽類(dialkylimidazolium)的離子液體熔點,如表二。[14]
- 黏度:由於正負離子的作用力,使得離子液體黏度通常比水的黏度大很多,離子液體黏度的大小受分子間的氫鍵及凡得瓦作用力影響,陽離子碳鏈愈長,凡得瓦力愈強則黏度愈高。對於相同種類的陽離子,不同陰離子所形成的離子液體其黏度高低順序為
Cl->PF6->BF4->NO3->(CF3SO3)2N-,如表二。
- 密度:大部分的離子液體是密度在 1 到 1.6 g/cm3之間,隨著溫度增加密度會降低。
離子液體在化學領域上有許多其特殊的物理性質,例如可溶解許多的無機和有機物質,但與部分的有機溶劑不互溶,可形成兩相反應系統,其優點是反應與分離可同時進行。不具揮發性,在高度真空下操作不易流失。不可燃性及有高的熱穩定性,加上其液體範圍廣,使其可應用的反應溫度範圍廣。另外,還可改變陰離子及陽離子的組成以調控其特性,所以離子液體亦被認為是“designer solvent”。離子液體的這些特殊物理性質,離子液體做為溶劑除了在電化學當作“nonaqueous elelctrolyte”外,其做為溶劑已被廣泛應用,其應用簡述如下:
基本上,隨著離子液體的研究發展,其應用將更深入、更廣泛,使其在綠色化學的重要性相對增加,但其邁向廣泛工業上應用仍有許多問題需要克服[57-58],此有待進一步研究來達成。
參考文獻:
- P. T. Anastas, J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A.
- K. R. Seddon, A. Stark, M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275.
- J. D. Holbery, K. R. Seddon, Clean Technol. Environ. Pol., 1999, 223.
- (a)P. Walden, Bull. Acad. Imper. Sci. (St. Petersburg), 1914, 1800; (b)S. Sugden and H. Wilkins, J. Chem. Soc., 1929, 1291.
- F. H. Hurley, T. P. Wier, J. Electrochem. Soc., 1951, 98, 203.
- H. L. Chum, V. R. Koch, L. L. Miller, R. A. Osteryong, J. Am. Chem. Soc., 1975, 97, 3264.
- J. S. Wilkes, M.J. Zaworotko, J. Chem. Soc., Chem. Commun. 1992, 965.
- J. H. Davis, P. A. Fox., Chem. Commun., 2003, 1209.
- A. M. Leone, S. C. Weatherly, M. E. Williams, H. H. Thorp, R. W. Murray, J. Am. Chem. Soc., 2001, 123, 218.
- M. Yoshizawa, A. Narita, H.Ohno, Aust. J. Chem. 2004, 57, 139.
- S. Hayashi, H. Hamaguchi, Chem. Lett., 2004, 33, 1590.
- K. Fukumoto, M. Yoshizawa, H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398.
- Y. Chauvin, H. Olivier-Bourbigou, CHEMTECH, 1995, 25, 26.
- R. Sheldon, Chem. Commun., 2001, 23991.
- P. Bonhote, A.-P. Dias, M. Papageorgiou, K. Kalyanasundaram, M. Gratzel, Inorg. Chem., 1996, 35, 1168.
- T. Welton, Chem. Rev., 1999, 99, 2071.
- D. J. Jaeger, C. E. Tucker, Tetrahedron lett., 1989, 30, 1785.
- D. Huchette, B. Thery, F. Petit, J. Mol. Catal., 1979, 4, 433.
- P. Wasserscheid, A. Bosmann, C. Bolm, Chem. Commun., 2002, 200.
- Y. Ishida, H. Miyauchi, K. Saigo, Chem. Commun., 2002, 2240.
- J.A. Boon, J.A. Levisky, J.L. Pflug, J.S. Wilkes J. Org. Chem. 1986, 51, 480.
- Y. Chauvin, L. Mussman and H. Olivier, Angew. Chem. Int. Ed. Engl., 1995, 34, 2698.
- E. G. Kuntz, Chemtech, 1987, 570.
- D. E. Kaufmann, M. Nouroozian and H. Henze, Synlett, 1996, 1091.
- D. Zim, R. F. de Souza, J. Dupont and A. L. Monteiro, Tetrahedron Lett., 1998, 39, 7071.
- J. Howarth, Tetrahedron Lett., 2000, 41, 6627.
- S. Park, R. J Kazlauaks, Curr. Opin. Biotecnol., 2003, 14, 432.
- D. W. Armstrong, L.-K. Zhang, L. He, M. L. Gross, Anal. Chem., 2001, 73, 3679.
- S. Carda-Broch, A. Berthod, D. W. Armstrong, Rapid Commum. Mass Spectrom., 2003, 17, 553 .
- C.F. Poole, K.G. Furton, B.R. Kersten, J. Chromatogr. Sci., 1986, 24, 400.
- S.K. Poole, C.F. Poole, J. Chromatogr., 1988, 435, 17.
- J. L Anderson,. D. W. Armstrong, Anal. Chem., 2003, 75, 4851.
- (a)D. W. Armstrong, L He, Y.-S. Liu, Anal. Chem., 1999, 71, 3873; (b)A. Berthod, L. He, D. W. Armstrong, Chromatographia, 2001, 53, 63.
- J. Ding, T. Welton, D. W. Armstrong, Anal. Chem., 2004, 76, 6819.
- G.-T. Wei, J.-C. Chen, Z. Yang, J. Chin. Chem. Soc., 2003, 50, 1123.
- G.-T. Wei, Z. Yang, C.-J. Chen, Anal. Chim. Acta, 2003, 488, 183.
- A.E. Visser, R.P. Swatloski, R.D. Rogers, Green Chem. 2000, 2, 1.
- W.-H. Lo, H.-Y. Yang, G..-T. Wei, Green Chemistry, 2003, 5, 639.
- P. Stepnowski, Anal. Bioanal. Chem., 2005, 381, 189.
- J.-F. Liu, G.-B. Jiang, Y.-G. Chi, Y.-Q. Cai, Q.–X. Zhou, J.–T. Hu, Anal. Chem., 2003, 75, 5870 .
- M. Andre, J. Loidl, G. Laus, H. Schottenberger, G. Bentivoglio, K. Wurst, K.-H. Ongania, Anal. Chem., 2005, 77, 702.
- G.-T. Wei, Z. Yang, C.-Y. Lee, H.-Y. Yang, C. R. C. Wang , J. Am. Chem. Soc., 2004, 126, 5036 .
- D. R. MacFarlane, J. Huang, M. Forsyth, Nature, 1999, 402, 792.
- M. Doyle, S. K. Choi, G. Proulx, J. Electrochem. Soc. 2000, 147, 34.
- W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada, S. Yanagida, Chem. Commun., 2002, 374.
- T. J. Boyle, D. Ingersoll, M, A. Rodriguez, C. J. Tafoya, D. H. Doughty, J. Electrochem. Soc., 1999, 146, 1687.
- M. C. Buzzeo,O. V.Klymenko,; Wadhawan, J. D.; Hardacre, C.; Seddon, K. R.; Compton, R. G. J. Phys. Chem. A, 2003, 107, 8872.
- I. M. AlNashef, M. L. Leonard, M. C. Kittle, M. A. Matthews, J. W. Weidner, Electrochem. Solid-State Lett., 2001, 4, D16.
- I. M. AlNashef, M. L. Leonard, M. A. Matthews, J. W. Weidner, Ind. Eng. Chem. Res., 2002, 41, 4475.
- D. Giovanelli, M. C. Buzzeo, N. S.Lawrence, C. Hardacre,; K. R. Seddon, R. G. Compton, Talanta, 2004, 62, 904.
- M. C. Buzzeo, C. Hardacre, R. G. Compton, Anal. Chem.2004, 76, 4583.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974.
- Y. Liu, M. Wang, Z. Li, H. Liu, P. He, J. Li, Langmuir, 2005, 21, 1618.
- C. F Ye, W. M. Liu, Y. X. Chen, L. G. Yu, Chem. Commun., 2001, 2244.
- Solvent-Innovation http://www.solvent-innovation.de.
- L. A. Blanchard, D. Hancu, E. J. Beckman, J. F. Brennecke, Nature, 1999, 399, 28.
- V.K. Aggarwal, I. Emme, A. Mereu Chem. Commun. 2002, 1612.
- P. Wasserscheid, T. Welton Ionic Liquids in Synthesis. Wiley-VCH: Weinheim, 2003.
表一、離子液體的陰離子與其酸鹼性
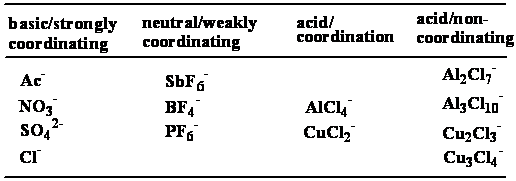
表二、各種dialkylimidazolium 鹽類的熔點及黏度


圖一、離子液體的結構圖 |
|
|
| 書籍 |
 |
Ionic Liquids in Synthesis (Hardcover) — by P. Wasserscheid
Wiley-VCH (January 29, 2007) |
 |
Metal Catalysed Reactions in Ionic Liquids (Catalysis by Metal Complexes) — by Paul J. Dyson, Tilmann J. Geldbach, Springer; 1 edition (January 9, 2006) |
 |
Electrochemical Aspects of Ionic Liquids (Hardcover) — by Hiroyuki Ohno, Wiley-Interscience (April 27, 2005)
|
 |
Ionic Liquids: Industrial Applications for Green Chemistry — by Robin D. Rogers (Editor)
|
 |
Ionic Liquids as Green Solvents: Progress and Prospects — by Robin D. Rogers (Editor), Kenneth R. Seddon (Editor) |
 |
Green Industrial Applications of Ionic Liquids —
by Robin D. Rogers (Editor), Kenneth Richard Seddon (Editor), Sergei Volkov (Editor) |
 |
The QUILL Handbook of Ionic Liquid — by Kenneth R. Seddon |
 |
Aqueous Biphasic Separations: Biomolecules to Metal Ions —
by Robin D. Rogers (Author), American Chemical Society Meeting (Author)1994 San Diego, Calif., Mark A. Eiteman (Editor) |
 |
Ionic Liquids Iiia: Fundamentals, Progress, Challenges, and Opportunities: Properties and Structure —
by Robin D. Rogers (Editor), Kenneth R. Seddon (Editor) |
| |
|
|
| Review |
 |
Design of Sustainable Chemical Products - The Example of Ionic Liquids — Chem. Rev. 2007, 107, 2183-2206. |
 |
Environmentally Benign Solvent Systems: Toward a Greener [4+2] Cycloaddition Process — Brummond KM, Wach CK Mini-Reviews in Organic Chemistry 4 (1): 89-103 FEB 2007. Abstract: Green chemistry is a field that encompasses a wide range of environmentally benign technologies. This review discusses the principles of green chemistry, as well as recent applications of these principles to the Diels-Alder reaction, with a focus on benign solvent systems. Specifically, Diels-Alder reactions in water, ionic liquids, supercritical carbon dioxide, and solvent-free systems will be reviewed up to February 2006.
|
 |
Ionic liquids. Green solvents for the future— Pure Appl. Chem., 2000, 72, 1391–1398
By Martyn J. Earle and Kenneth R. Seddon.The QUILL Centre, Stranmillis Road, The Queen’s University of Belfast, Northern Ireland, BT9 5AG, UK |
 |
Room-temperature Ionic Liquids. Solvents for Synthesis and Catalysis — Chem. Rev., 1999, 99, 2071
|
 |
A Short History of Ionic Liquids—From Molten Salts to Neoteric Solvents — Green Chem., 2002, 4, 73 An excellent short history of ILs has been presented by eyewitness to and participant in crucial developments, professor John S. Wilkes.
|
 |
Ionic Liquids: Solvent Properties and Organic Reactivity — J. Phys. Org. Chem. 2005, 18, 275.
|
| Catalysis Synthesis |
 |
Room-temperature Ionic Liquids. Solvents for Synthesis and Catalysis — Chem. Rev. 1999, 99, 2071.
1999年離子液體中催化回顧文章。 |
 |
New Developments in Catalysis Using Ionic Liquids — Applied Catalysis A: General 2001, 222, 101.
2001年離子液體中催化回顧文章。 |
 |
Ionic Liquids: Applications in Catalysis — Catalysis Today 2002, 74, 157.
2002年離子液體中催化回顧文章。 |
 |
Ionic Liquid (Molten Salt) Phase Organometallic Catalysis — Chem. Rev. 2002, 102, 3667. 2002年離子液體中催化回顧文章。 |
 |
Ionic Liquids in Catalysis — Coord. Chem. Rev.2004, 248, 2459.
2004年離子液體中催化回顧文章。 |
 |
Ionic Liquids: Green Solvents for Nonaqueous Biocatalysis — Enzyme Microb. Technol. 2005, 37, 19.
離子液體在生物催化中的回顧。 |
 |
Chiral Ionic Liquids: Synthesis and Applications — Chirality 2005, 17, 281.
Chiral離子液體合成與應用的回顧。 |
 |
Ionic Liquids as Solvents for Catalyzed Oxidations of Organic Compounds — Adv. Synth. Catal. 2006, 348, 275.
以離子液體為溶劑的氧化反應回顧。 |
Analytical Chemistry |
 |
Chromatographic and Spectroscopic Methods for the Determination of Solvent Properties of Room Temperature Ionic Liquids — J. Chormatogr. A, 2004, 1037, 49 |
 |
An Analytical View of Ionic Liquids — Analyst, 2005, 130, 800 |
 |
Application of Ionic Liquids in Analytical Chemistry — Trends Anal. Chem., 2005, 24, 20 |
 |
Ionic Liquids in Chemical Analysis — Crit. Rev. Anal. Chem., 2005, 35, 177 |
 |
Analytical Applications of Room-temperature Ionic Liquids: A Review of Recent Efforts — Anal. Chim. Acta, 2006, 556, 38 |
| Electrochemistry |
 |
Ionic Liquids: Solvents for the Electrodeposition of Metals and Semiconductors — ChemPhysChem 2002, 3 (2): 144. |
| |
|
|
| Articles |
| Synthesis and Catalysis |
| Analytical Chemistry |
|
Separations |
| |
|
Capillary electrophoresis |
| |
|
Extraction |
| |
|
GC |
| |
|
LC |
|
Mass Spectrometry. |
| |
Solvent for spectroscopy |
|
Others |
| Toxicity |
 |
Effects of different head groups and functionalized side chains on the aquatic toxicity of ionic liquids — 如何降低離子液體之毒性 ? Green Chem., 2007, 9, 1170-1179. New(2008.5.7) |
 |
On the Freshwater Ecotoxicity and Biodegradation Properties of Some
Common Ionic Liquids - Organic Process Research and Development 2006, 10,
794. — It is suggsted that ionic liquids can be ecotoxic. Contamination of aqueous effluent streams by these materials should be avoided. |
| Taiwanese researchers' papers |
 |
國立中正大學化學暨生物化學系, 朱延和 — Tetrahedron Letters 2006, 47, 1575. |
 |
國立中正大學化學暨生物化學系, 魏國佐 — J. Am. Chem. Soc. 2004, 126(16), 5036. |
 |
國立成功大學大學化學系, 孫亦文 —Tetrahedron 2005, 61, 4857. |
| |
|
|
more.. |
| |
|
|
|
|
| Conferences |
 |
226th ACS Meeting— IL section, 226thACS meeting in New York, NY, Spetember 7-11, 2003 |
| |
|
|
| 相關網站 |
|
| Ionic Liquid Groups |
|
| |
| |
|
| |
|
聯絡我們|國科會化學中心 |
| 本站適用瀏覽器為IE 6.0以上;最佳瀏覽解析度為1024*768 Update:
2020-09-08
|
| Copyright©2006 國科會化學中心. All Rights Reserved |
|
|
 |



